Fluorescence imaging with short-wave infrared light has the potential to significantly increase precision and safety in cancer surgery. So far, however, there are very few clinically applicable dyes for this purpose. An international team of researchers led by scientists at the National Cancer Institute (Bethesda, Maryland, USA) and Helmholtz Zentrum München has now developed two new fluorescent dyes for the short-wave infrared range and an associated imaging system.
This opens up the possibility for the first time to visualize certain structures — such as the tumor, the surrounding healthy tissue and draining lymphatic vessels — simultaneously in a liquid video image during surgery. After his appointment at the National Center for Tumor Diseases Dresden (NCT/UCC), Prof. Oliver Bruns will further develop the innovative imaging method for cancer surgery in Dresden. The results were published in the renowned journal Nature Methods.
The National Center for Tumor Diseases Dresden (NCT/UCC) is a joint institution of the German Cancer Research Center (DKFZ), the Carl Gustav Carus University Hospital Dresden, the Carl Gustav Carus Medical Faculty of TU Dresden and the Helmholtz-Zentrum Dresden-Rossendorf (HZDR).
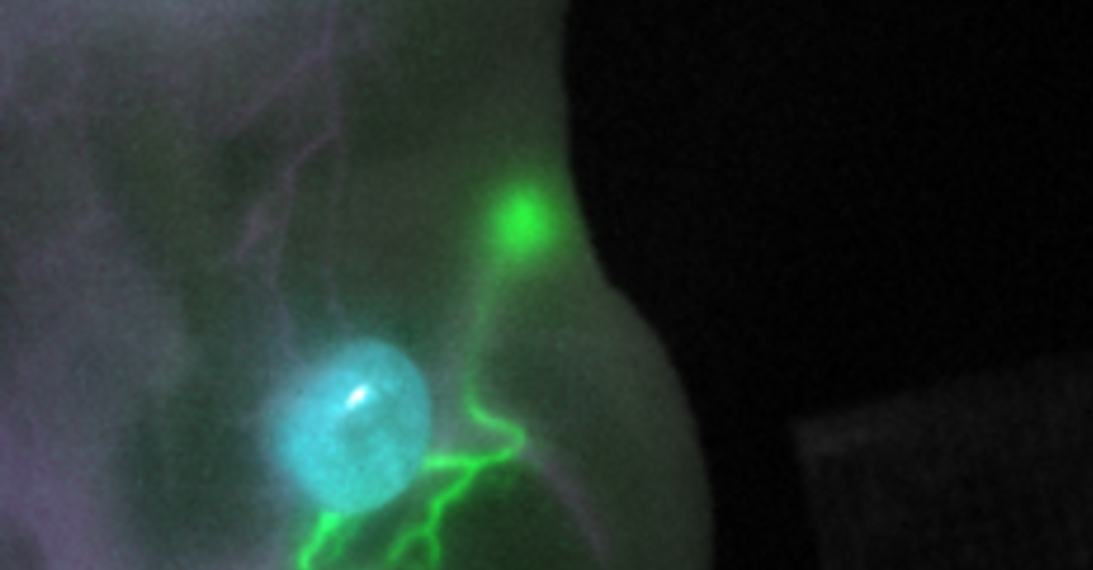
Tumor operations are millimeter work. The goal is to completely remove the tumor and possible metastases. At the same time, healthy tissue and surrounding risk structures such as nerves and vessels must be spared as much as possible. Fluorescence imaging with short-wave infrared light (SWIR) with wavelengths greater than 1,000 nanometers is intended to support surgeons in this difficult task in the future.
Using SWIR, significantly sharper images and insights into deeper tissue layers can be achieved than with conventional fluorescence imaging, which works with infrared light in the wavelength range of 700 to 900 nanometers. In addition, the innovative method offers the potential to simultaneously illuminate selected tissues, vessels and body fluids in a targeted manner. Now that prices for suitable cameras have dropped significantly due to rapid developments in the industry, suitable dyes are the crucial bottleneck for medical applications.
“The advances in dye and technology development that have now been achieved create the necessary conditions for the first time to visualize various structures such as the tumor, draining lymphatic vessels and sentinel lymph nodes in a very dynamic process such as fluorescence-guided surgery.”
- Prof. Oliver Bruns
Bruns was appointed professor of Functional Imaging in Operative Oncology at the National Center for Tumor Diseases Dresden (NCT/UCC) in February in a joint appointment by the TU Dresden and the German Cancer Research Center (DKFZ) and was also appointed group leader at Helmholtz Zentrum München. The research results on fluorescence imaging achieved during his time at Helmholtz Zentrum München are an important basis for future research in Dresden.
For dye development, the researchers relied on cyanine dyes, which are among the most common dyes for multicolor fluorescence microscopy. However, only a few of them are suitable for imaging in living organisms and in the SWIR range. Fluorescent dyes are usually administered intravenously and some can be linked to antibodies that allow them to bind specifically to tumors. Targeted computer-assisted enhancement of a clinically approved class of molecules led to the development of two new dyes (FNIR-872, FNIR-1072) that emit most of their light in the SWIR range after optical excitation. “Other key properties of the newly developed dyes are that they are nontoxic, dissolve well in water, and can couple with biomolecules that transport the dyes to the desired targets in the organism,” explains Martin Schnermann, Ph.D., of the National Cancer Institute.
In parallel with the dyes, the researchers developed an imaging system with three lasers and an LED source. This makes it possible to simultaneously excite an already approved dye suitable for SWIR imaging (indocyanine green, ICG) as well as the two newly developed dyes with the respective suitable wavelengths. The different image information can be bundled into a common view and converted into a smooth video image at a rate of eight frames per second. This opens up the possibility of providing surgeons with a continuous view of all important target and risk structures during an operation. Previous fluorescence imaging, on the other hand, only allowed individual structures to be highlighted in lower quality at a time. The newly developed imaging system also has the advantage that it is not sensitive to visible light and enables operations to be performed under normal room lighting conditions.