An important role in the study of mRNA nanoparticles is played by the Heinz Maier-Leibnitz Research Neutron Source (FRM II) at the Technical University of Munich (TUM), similar to those used in the Covid-19 vaccine of the companies BioNTech and Pfizer. Using the high neutron flux available in Garching, researchers at the Heinz Maier-Leibnitz Center (MLZ) were able to characterize different formulations for the mRNA vaccine, providing a basis for improving their efficacy.
Using messenger RNA (mRNA) as an active substance is in principle an ingenious idea: the molecule contains the specific blueprint for proteins that are then synthesized by the cell. This means that, in principle, a very wide range of different therapeutically active proteins can be made available.
In the case of the Covid-19 vaccine, these are the proteins of the characteristic spikes on the surface of the corona virus that are used for vaccination. These are presented on the surface of immune cells whereupon the human immune system initiates defense against these foreign proteins and thus against the corona viruses. The mRNA itself is completely degraded after a few hours, which is advantageous for the safety of these vaccines.
The way to the best packaging
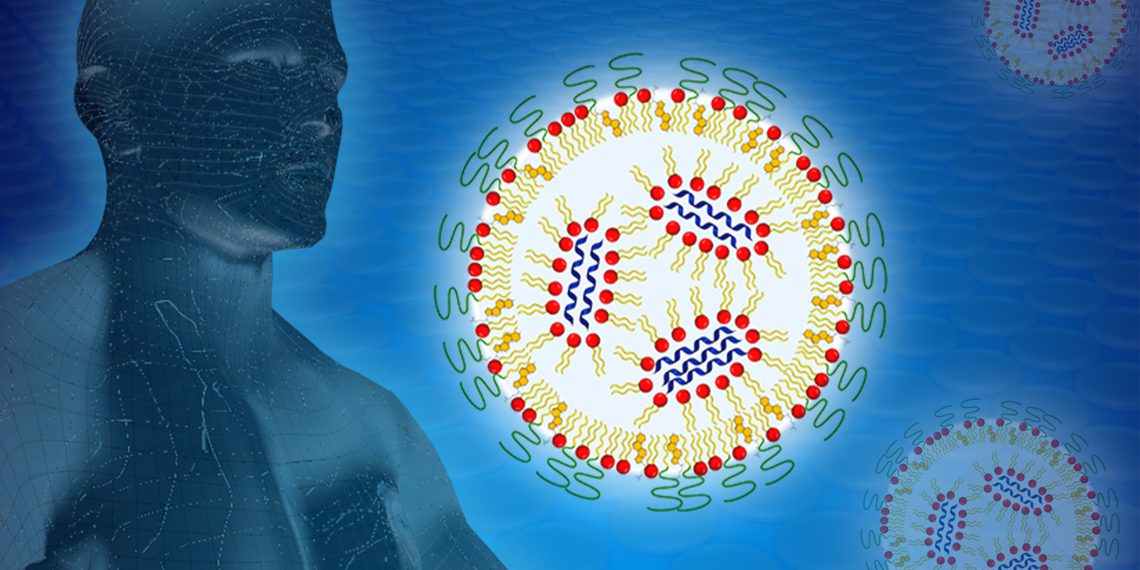
To prevent the mRNA from being degraded by the body’s ubiquitous enzymes on its way to the cell, it must be packaged appropriately. This is done by nanoparticles, which can be a mixture of lipids or polymers.
Lipids are fatty molecules that resemble cell envelope molecules and help deliver mRNA into the cell interior. Lipids and biopolymers are subsequently degraded or excreted by the body.
In collaboration with the group of Prof. Peter Langguth, Department of Pharmaceutical Technology at the Institute of Pharmaceutical and Biomedical Sciences at Johannes Gutenberg University in Mainz, Germany, the BioNTech formulation team, led by Dr. Heinrich Haas, developed a series of formulations for this purpose in which the nanoparticles consisted of lipids and biopolymers in various mixtures that had already been tried and tested in pharmaceuticals.
Fluoroscopy with neutrons
To compare the properties of differently composed nanoparticles, the researchers subjected them to a wide variety of tests. In addition to X‑ray and microscopic analyses, these included irradiation with neutrons at the KWS‑2 instrument operated by Forschungszentrum Jülich at FRM II of TU München in Garching.
The neutrons are scattered by the hydrogen nuclei inside the nanoparticles and deflected from their path in characteristic ways. From this, conclusions can be drawn about their distribution. If the hydrogen atoms of certain components — for example, only of the lipids — are now exchanged for heavy hydrogen, the chemical properties or the pharmaceutical effect do not change, but the scattering of the neutrons does.
“With this method, parts of a complex multicomponent morphology can be selectively highlighted without changing the physical chemistry of the sample,” says Dr. Aurel Radulescu of the Jülich Centre for Neutron Science (JCNS), who is responsible for the KWS‑2 instrument and led the analysis of the measurement results. “In this way, structural properties can be visualized that are not or hardly visible with other methods.”
The right amount of order does the trick
In these analyses, the research teams were interested in how efficiently the transfer of mRNA into the cell, known as transfection, worked for the different formulations. In this way, the researchers found that the highest transfection rate was obtained with nanoparticles characterized by a certain type of internal order.
“Whenever ordered and less ordered regions inside the nanoparticles alternated in a characteristic way, high biological activity was observed. This could be a general concept of the structure-activity relationship that is applicable independently of the systems studied here.”
- Dr. Heinrich Haas
An improved process
To obtain the desired structural properties, lipids and biopolymers had to be brought together with the mRNA using precisely defined procedures. In the process, the research team was able to show that the nanoparticles used to package the mRNA could be produced in a single step, a significant simplification compared to a two-step process that was also originally tested.
Thus, in the end, a simplified method for producing mRNA nanoparticles with improved activity was found. “Such issues of practical manufacturability represent an important prerequisite for the developability of pharmaceutical products,” Prof. Langguth clarifies. In the future, such concepts may be considered in the development of new mRNA-based therapeutics.
The work was funded by the German Federal Ministry of Education and Research (BMBF), the German Research Foundation (DFG) in the framework of the Collaborative Research Center SFB 1066, the Max Planck Graduate Center (MPGC-Mainz) and the Lewis Trust.
The Small Angle Neutron Scattering (SANS) experiments were performed at the KWS‑2 instrument at the FRM II research neutron source at the Technical University of Munich. As part of the Heinz Maier-Leibnitz Center, the instrument is operated there by the Jülich Centre for Neutron Science.
Small Angle X‑ray Scattering (SAXS) experiments were performed at the P12 beamline operated by the European Molecular Biology Laboratory (EMBL) at the PETRA III synchrotron radiation source of the German Electron Synchrotron (DESY) in Hamburg.