Iridium-catalyzed hydrogenation to produce natural products from plants and insects
Crucial to the cost of synthesizing pharmaceuticals is how much waste is produced. A research team has now found a catalyst that allows hydrogen to be added to carbon-carbon bonds with exceptionally high accuracy. In the journal Angewandte Chemie, the authors:in describe the reaction as a helpful substep in the production of complicated natural products such as pheromones.
Nature has developed an arsenal of natural products, and many of them proved to be effective medicines. For example, plant natural products such as polyketides and pheromones have great potential as antitumor drugs and antibiotics. Many pharmaceutically active substances are effective in only one of two configurations (they may even be harmful in the other). To generate the right product, either detours must be taken, such as synthesizing both options and discarding one, or using a suitable catalyst to specifically produce only one version.
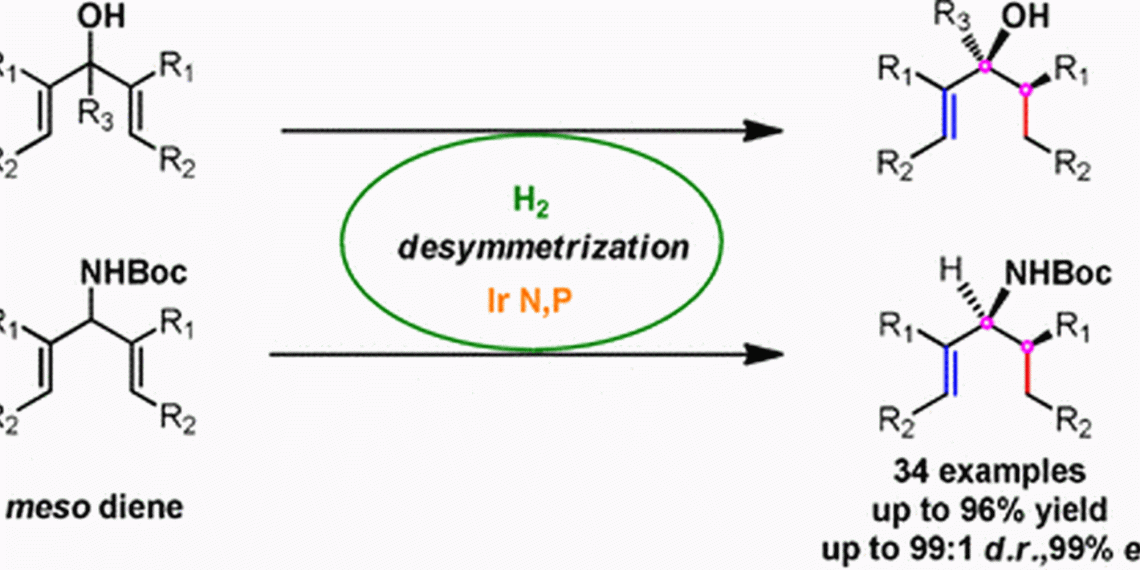
Pher G. Andersson and his team at Stockholm University have discovered that a catalyst consisting of the heavy metal iridium and organic phosphorus-nitrogen units is very effective at hydrogenating symmetrical organic compounds. Hydrogenation is the addition of hydrogen to unsaturated carbon-carbon bonds. The reaction is considered particularly economical because no by-products are formed, but also particularly critical because at the moment of hydrogenation the configuration, i.e. the handedness, of the product is decided.
Symmetric unsaturated compounds are often precursors for polyketides and pheromone-like natural products. With the iridium catalyst, it was now possible to specifically hydrogenate one of the symmetric carbon-carbon bonds. “The method is the first example of iridium-catalyzed hydrogenative desymmetrization of dienes,” the authors write. They demonstrated how useful the new method can be by using dozens of precursors, which they converted to the desired products with almost no byproducts.
The target configuration at the oxygen group in the vicinity of an unsaturated bond was important. These so-called allylic carbinole are frequently found in natural products. Others contain nitrogen groups, the so-called allylic carbamines. Whether nitrogen or oxygen, in both cases the iridium catalyst provided the correct configuration, the authors write. Another common structural motif of natural products is lactones. Here, too, the iridium catalyst did well, and the researchers were able to find a simple synthetic route through hydrogenative desymmetrization.
Using the new method, the authors completely synthesized two natural products, one being zaragozaic acid, a polyketide derived from fungi, and the other being the ant pheromone invictolide. The method’s high selectivity and almost complete preference throughout for a configuration that yields the product with the right configuration and handedness make it an economical and versatile alternative for the synthesis of important pharmaceutical products, the authors say.