A living cell is exposed to a variety of stimuli. Countless messenger substances dock onto its surface, transmit their messages and trigger signals inside the cell. As a result, the cell changes its functions, its metabolism or switches genes in the cell nucleus on or off. Receptors in the cell membrane accept the information for all these orders. The messages from the outside world are received by many different receptors located in the cell membrane. But how does the cell manage to distinguish between the signals of different receptors? A team led by pharmacologist Martin Lohse and including researchers from the Max Delbrück Center in Berlin, the University of Würzburg and ISAR Bioscience has now demonstrated that previously unknown nanodomains play a crucial role.
There are more than 800 different receptors located on the cell surface. Up to a hundred different receptor types can be located on a single cell, and these in turn respond to very different messenger substances. “Countless signals come from outside, which are recognized quite specifically by receptors — but in the cell there are only a handful of molecules that respond to the activation. Yet they perform diverse and completely different tasks,” says Andreas Bock. A long-time collaborator of Martin Lohse, he has been a professor at the University of Leipzig since the beginning of 2022 and is the last author of the recently published study in the renowned journal “Cell.”
Communication in nanometer-sized spaces
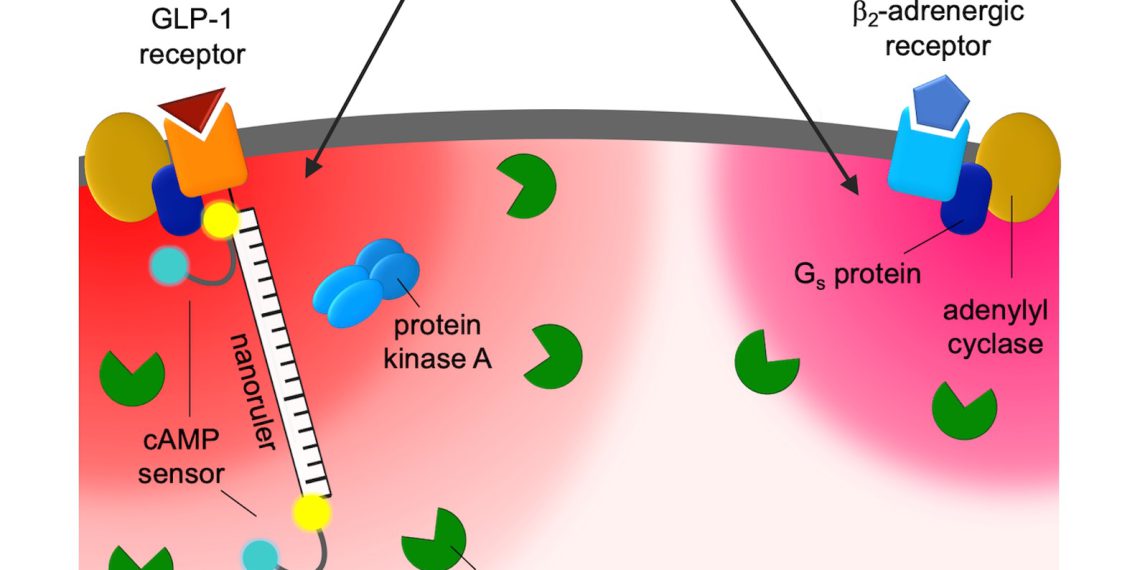
Cyclic adenosine monophosphate (cAMP) is the most important signaling molecule in the cell. It is produced when certain receptors are stimulated. For example, if heart muscle cells are stimulated with adrenaline, their cAMP level increases and the heart contracts faster and more forcefully. If the same cells are stimulated with prostaglandin, the same amount of cAMP is produced, but surprisingly the heart muscle hardly reacts at all.
Using fluorescence microscopy, the researchers used isolated single cells to investigate how cAMP signals from two different receptors are generated and processed in parallel in one cell. They realized that under normal conditions, the increase in cAMP levels is limited to tiny domains directly at the activated receptor with a radius between 30 and 60 nanometers. “These are compartmentalized spaces in which the cAMP concentration is very high — it is in them that the different effects of cAMP arise,” explains Andreas Bock “We assume that the high specificity of receptor stimuli arises via the narrow localization of the nanospaces. We have named these small spaces RAINs: Receptor-associated independent nanodomains.”
“The discovery of nanodomains increases the complexity of signaling pathways in the cell many times over what we previously thought,” said Charlotte Kayser, Ph. Together with Dr. Selma Anton, she is first author of the study. Signals that originate at the receptor first remain on site and only influence the enzymes in the immediate vicinity. Other areas in the cell are therefore not addressed by the signals. This means that individual signaling pathways can be switched on and off very locally.
For a long time, scientists regarded the cytosol, the interior of the cell, as a large “swimming pool” in which cell components cavort freely. But there appear to be previously unknown structures that organize the cell interior around each receptor. “We cannot see the nanospheres directly — they are too small even for the best light microscopes,” explains senior author Professor Martin Lohse, former director of the Max Delbrück Center and now chairman of ISAR Bioscience.
Cells can process large amounts of signals in parallel
The cell does not appear to be a switch that is either “on” or “off. It functions more like a chip in which many signals are processed simultaneously in a very small area, says Lohse. “This is very important for nerve cells, for example, which can each process different signals at their extensions in this way. One site can be activated, while another is dormant and a third is inhibited.”
Once the scientists stimulated a cell with small amounts of messenger — hormone or neurotransmitter — the nanodomains were strongly expressed. With stronger stimulation, the signaling molecules “spilled over” and the spaces began to merge. This could have medical applications. “It may be possible to produce not only quantitatively but also qualitatively different effects with substances that stimulate receptors to different degrees — I’m thinking of opioids, for example. Depending on whether the triggered cAMP signals affect only individual regions of the cell or encompass the entire cell,” adds Martin Lohse.
“We have provided a first glimpse of a previously unimagined nanoworld within cells. With research funding from the European Research Council, we’ve been looking for a ‘quantum world’ of cellular signaling since 2008 — now we can say it really exists.”
- Martin Lohse
The first task is to better understand the structure and components of such nanodomains. However, initial findings already show that they no longer function properly in diseased cells, such as liver cancer cells or in the diseased heart. This makes the cellular nanoworld interesting for medicine as well.