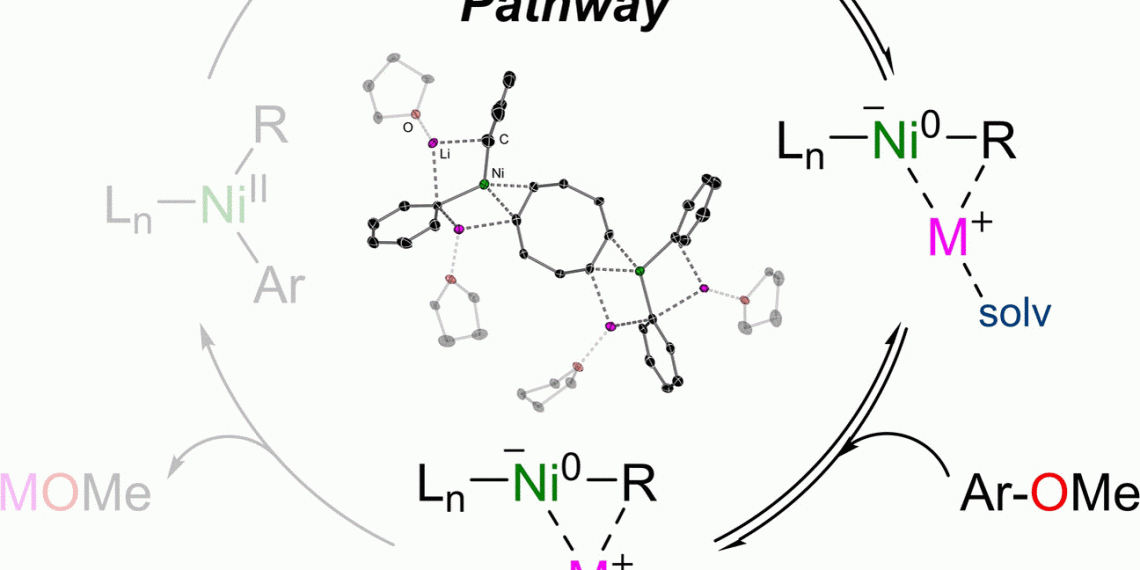
Researchers have now discovered a special property of nickel as a catalyst. This metal catalyzes the linkage of aromatic hydrocarbons as a nickelate, a negative ion. In this ion, the two metals lithium and nickel are in an unusual relationship, according to the publication in the journal Angewandte Chemie.
When building carbon skeletons, chemists:inside like to rely on catalysts made of the noble metal palladium. Its parade reaction is cross-coupling, in which two hydrocarbon fragments are joined together to form a larger unit. However, some substrates resist the reaction. Then detours must be taken or the substrates must first be activated for such coupling reactions by certain tricks.
In some cases, nickel could provide a remedy as an alternative catalyst, write Eva Hevia and Andryj M. Borys of the University of Bern. They showed that under certain conditions, nickel forms a negatively charged intermediate that also causes otherwise rather sluggish substrates to react.
For example, a nickel catalyst catalyzes the cross-coupling of aryl ethers, which are found in tar and other petroleum products and are basic materials for many specialty chemicals. Such substances are not very reactive, so they often have to be laboriously activated for reactions. Hevia and Borys now studied the reaction of phenyllithium (activated benzene) with beta-naphthymethyl ether, a simple aryl ether. They used a nickel catalyst consisting of one nickel atom and two molecules of cyclooctadiene, abbreviated as Ni(COD)2.
The researchers discovered that the negatively charged ion, or nickelate, formed in the very first reaction step. Two molecules of phenyllithium transferred their negatively charged phenyl radical to the neutral nickel atom. This was only possible, the team said, because the lithium ions and coordinated solvent molecules stabilized the buildup of the nickelate.
As the reaction progressed, the nickelate catalyst initiated the cleavage of the difficult ether bond between carbon and oxygen, after which the product, phyenylnaphthalene, could form. The authors identified the stabilizing solvent and the cooperation between lithium and nickel as instrumental in the success of the reaction.
The anionic reaction pathway offers an interesting alternative to the commonly used palladium-catalyzed cross-couplings. However, as Borys’ and Hevia’s work shows, this reaction pathway depends on how well the catalyst forms and how stable it is.
“The insights into the reaction mechanism made in this study will allow further development of nickel catalysis for sustainable syntheses.”
- Eva Hevia