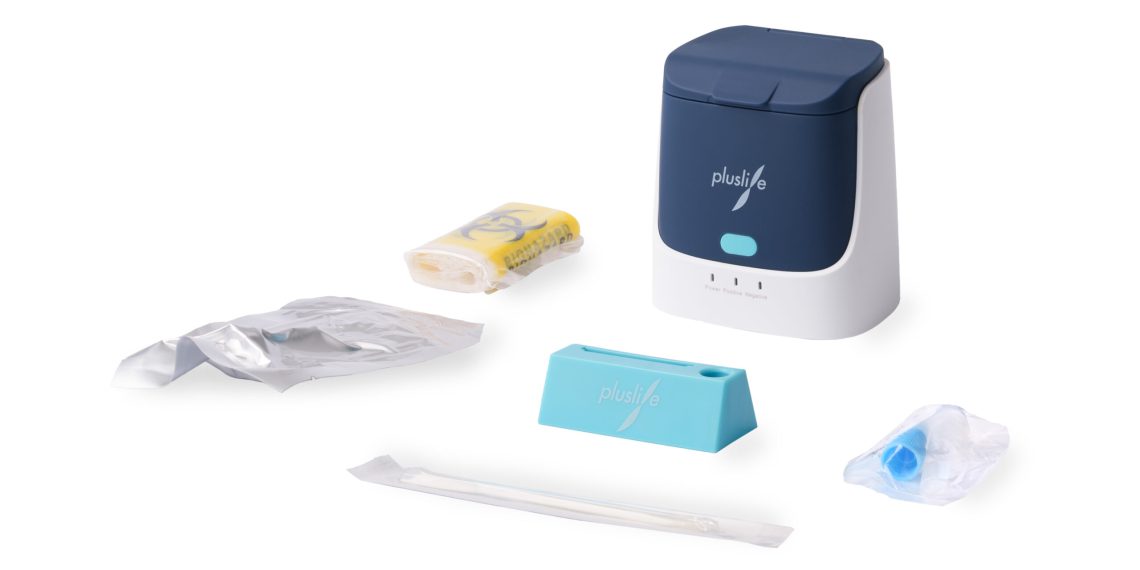
MedRhein GmbH has already become a pioneer in the import of in vitro diagnostics through its range of point-of-care PCR devices. In the search for innovative medical technology that can also be operated without trained specialists, they found what they were looking for in the NAT device pluslife Mini Dock. The device, which weighs only 210g and is particularly handy, has already been undergoing rigorous field testing by major customers for several months, after the manufacturer’s specifications on specificity and sensitivity were confirmed by the MedRhein laboratory team.
The test procedure of the pluslife Mini Dock is based on an isothermal amplification method (RHAM). This is also a NAT (nucleic acid amplification technique) procedure like the PCR test. The comparability to PCR devices is also confirmed by the reimbursement according to §9 of the test regulation and the accuracy according to manufacturer’s data and laboratory tests.
In addition, pluslife Mini Dock is particularly attractive due to its low price in direct comparison to PCR devices. Pharmacies and nursing homes, for example, are now given the opportunity to perform PCR-quality corona tests themselves on site — with a rapid result within 15–35 minutes.